FISMA the Framework for Information Specification, Modelling and Architecture, is currently tailored for Duchenne and Becker muscular dystrophies
FISMA has been integrated into LUMC’s electronic health record system HiX, the biobank management system Sample Navigator, and the data capture platform Castor EDC.
It is utilized in the Dutch Dystrophinopathy Database (DDD)—a national registry for patients in the Netherlands with Duchenne, Becker and females carrying a pathogenic DMD variant—as well as in the LUMC NeuroMuscular Diseases biobank.
FISMA has a multi-faceted approach with several underlying principles:
- Data-elements embedded in their clinical context (real-world data)
- Registered once -at the source- in a clinical setting (integration of research and healthcare)
- Unambiguously defined with internationally adopted ontologies, standards
- Data elements are referenced in and curated for standards of care
- Wherever applicable, data elements were associated with relevant protocols
- System-independent, allowing implementation in any data capture solution
- Data is reusable for multiple purposes
- Expandable without disrupting existing implementations.
- Expandable to other neuromuscular diseases
So the core principles of FISMA are: Context is key; FAIR*- from the start; Registration at the source
*FAIR: Findable, Accessible, Interoperable, Reusable
By richly embedding each data element in metadata, context is provided that allows Real-world-data to be reused for multiple purposes.
More information:
The DDD
The Dutch Dystrophinopathy Database, the DDD, is a national registry for patients in the Netherlands with Duchenne muscular dystrophy, Becker muscular dystrophy and females presenting a pathogenic DMD variant. DDD was setup in 2008 and in 2018 the newest iteration of the database was established.
DDD captures both patient- and clinicians reported healthcare data
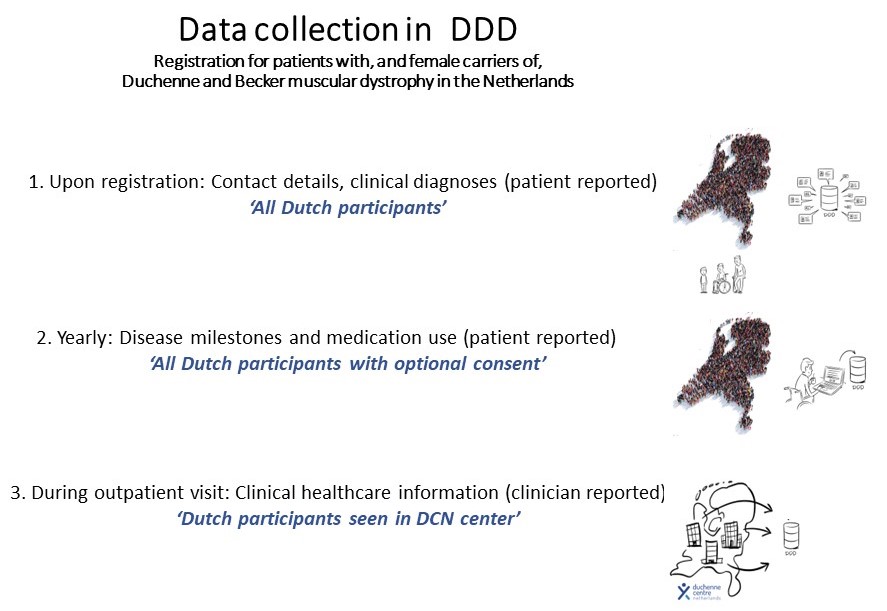
DDD is used as a platform to (1) select eligible study candidates, (2) perform epidemiology studies of dystrophinopathies, (3) collect longitudinal real-world data for e.g. natural history studies, (4) facilitate feasibility studies, (5) perform post-market surveillance and (6) collaborate in (inter)national data-driven initiatives.
DDD facilitates:
- Clinical trial readiness
- Post-marketing surveillance
- Effective use and interoperability of real-word healthcare data
- Reduced registration burden for patients and healthcare providers.
The design of the newest DDD iteration is based on a system-independent information model (FISMA) that adheres to international standards, and has been drafted with registration at the source, interoperability and re-use ability of data in mind. To maximize enrollment, patients can provide consent online and participate in the registry on different levels. The governance and access of data is covered and includes a general board, an advisory board, database management, and genetic surveillance.
DDD: the historical background
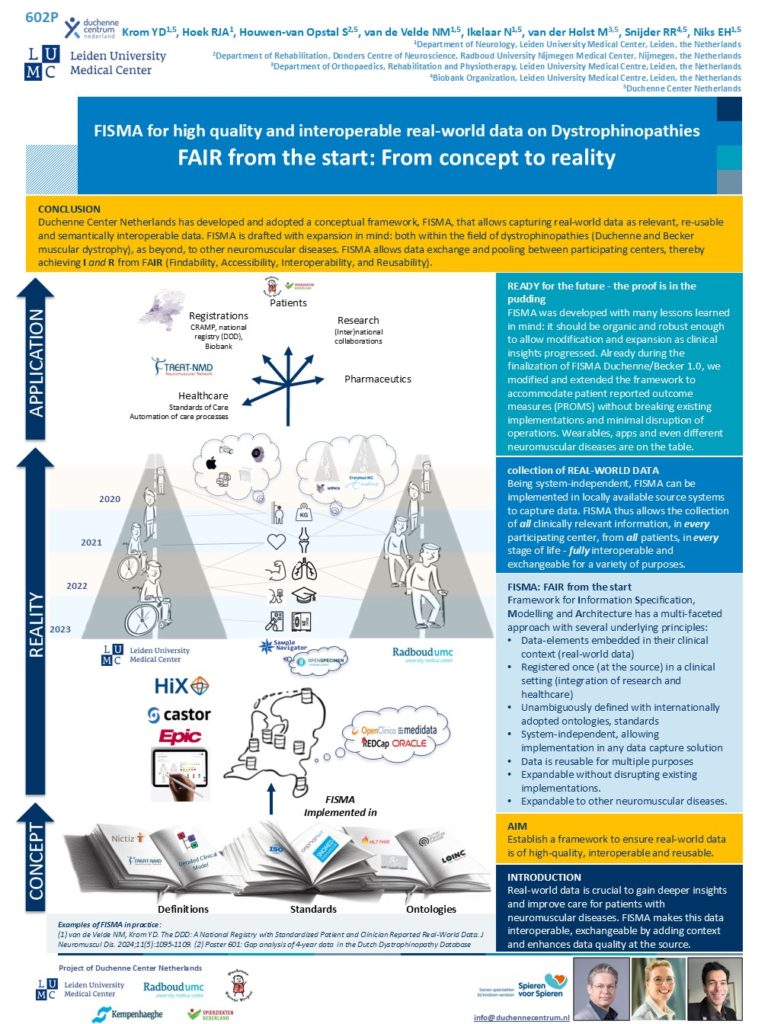
2008: The Dutch Dystrophinopathy Database version 1 was originally set up as a stand-alone database on a single computer at the Leiden University Medical Center (LUMC).
2014: The second iteration, DDD2, was established on a web-based database management system that was developed at the LUMC.
2016: The Duchenne Centre Netherlands (DCN) was founded as collaboration between three academic partners (LUMC, Radboudumc and Kempenhaeghe-MaastrichtUMC+) and the two patient organizations Duchenne Parent Project and Spierziekten Nederland with financial support from Spieren voor Spieren foundation. Objectives were to increase trial readiness, to facilitating multicenter collection of longitudinal real-world data and to improve governance and quality control.
2018: The third iteration, DDD3, was established. The design of DDD3 is based on FISMA; Framework for Information Specification, Modelling and Architecture. FISMA has a multi-faceted approach with several underlying principles. See info into FISMA- the information model
Hier nog info over de history; van PIM-PRISMA naar FISMA, met mogelijk links naar papers!
DDD: enrolment, data flow and governance structure
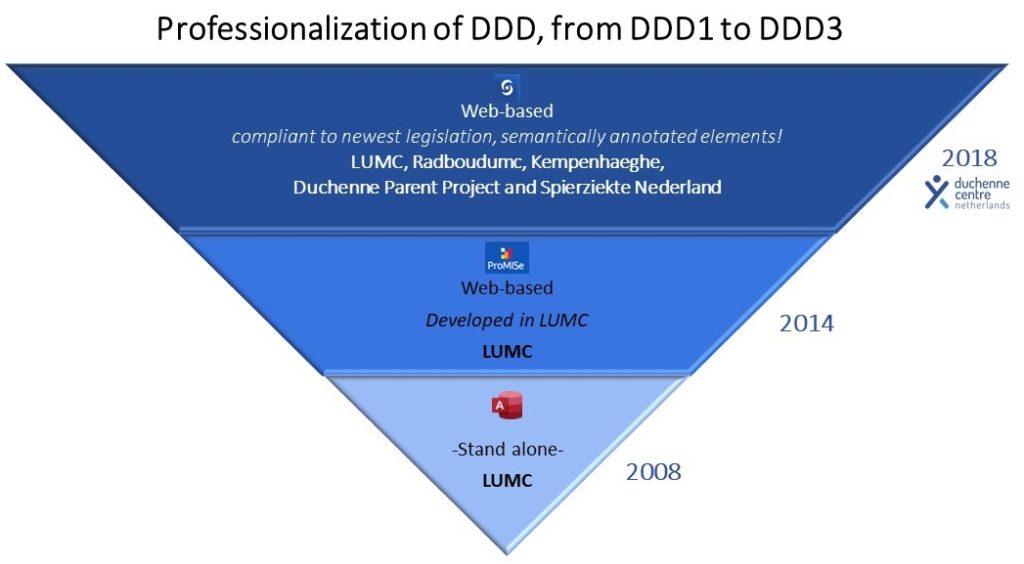
To maximize enrolment, patients can provide consent online and participate in the registry on different levels.
The minimal requirement for registration is (1) consent to provide contact details, the clinical diagnosis, and for the database manager to request genetic test results. In addition, participants can choose to (2) provide information on disease milestones and medication via a yearly online questionnaire, and (3) to retrieve and store their clinical data if their outpatient visit is performed in one of the Duchenne expert centers, so within the Leiden University Medical Center (LUMC), Radboudumc and/or Kempenhaeghe.
Governance structure
the DDD is managed by the Duchenne Center Netherlands. They have a governance structure in place.
A collaboration agreement between the five partners, LUMC, Radboudumc, Kempenhaeghe-MUMC+, and the two patient organizations Duchenne Parent Project and Spierziekten Nederland, that described the allocation of tasks and responsibilities related to the design, management and use of DDD is drawn and a DDD general board, a DDD advisory board and a structure to access the data is in place.
The DDD general board consists of representatives of each of the five aforementioned partners. The DDD advisory board consist of independent representatives from each of the five DCN partners. The advisory board is a consultative body, making solicited and unsolicited recommendations on scientifically relevant aspects regarding the purpose and use of DDD3, and evaluates data requests from researchers to provide an advice to the DDD general board.
To gain access to data, any researcher, regardless whether they are employed by one of the partners of Duchenne CN, or employed elsewhere, has to provide a written application study rationale, research question(s), study design and methodology.
DDD registry team
Contact: Registration@duchennecentrum.nl
LUMC NMD Biobank
The DDD
The Dutch Dystrophinopathy Database, the DDD, is a national registry for patients in the Netherlands with Duchenne muscular dystrophy, Becker muscular dystrophy and females presenting a pathogenic DMD variant. DDD was setup in 2008 and in 2018 the newest iteration of the database was established.
DDD captures both patient- and clinicians reported healthcare data
DDD is used as a platform to (1) select eligible study candidates, (2) perform epidemiology studies of dystrophinopathies, (3) collect longitudinal real-world data for e.g. natural history studies, (4) facilitate feasibility studies, (5) perform post-market surveillance and (6) collaborate in (inter)national data-driven initiatives.
DDD facilitates:
- Clinical trial readiness
- Post-marketing surveillance
- Effective use and interoperability of real-word healthcare data
- Reduced registration burden for patients and healthcare providers.
The design of the newest DDD iteration is based on a system-independent information model (FISMA) that adheres to international standards, and has been drafted with registration at the source, interoperability and re-use ability of data in mind. To maximize enrollment, patients can provide consent online and participate in the registry on different levels. The governance and access of data is covered and includes a general board, an advisory board, database management, and genetic surveillance.
DDD: the historical background
2008: The Dutch Dystrophinopathy Database version 1 was originally set up as a stand-alone database on a single computer at the Leiden University Medical Center (LUMC).
2014: The second iteration, DDD2, was established on a web-based database management system that was developed at the LUMC.
2016: The Duchenne Centre Netherlands (DCN) was founded as collaboration between three academic partners (LUMC, Radboudumc and Kempenhaeghe-MaastrichtUMC+) and the two patient organizations Duchenne Parent Project and Spierziekten Nederland with financial support from Spieren voor Spieren foundation. Objectives were to increase trial readiness, to facilitating multicenter collection of longitudinal real-world data and to improve governance and quality control.
2018: The third iteration, DDD3, was established. The design of DDD3 is based on FISMA; Framework for Information Specification, Modelling and Architecture. FISMA has a multi-faceted approach with several underlying principles. See info into FISMA- the information model
Hier nog info over de history; van PIM-PRISMA naar FISMA, met mogelijk links naar papers!
DDD: enrolment, data flow and governance structure
To maximize enrolment, patients can provide consent online and participate in the registry on different levels.
The minimal requirement for registration is (1) consent to provide contact details, the clinical diagnosis, and for the database manager to request genetic test results. In addition, participants can choose to (2) provide information on disease milestones and medication via a yearly online questionnaire, and (3) to retrieve and store their clinical data if their outpatient visit is performed in one of the Duchenne expert centers, so within the Leiden University Medical Center (LUMC), Radboudumc and/or Kempenhaeghe.
Governance structure
the DDD is managed by the Duchenne Center Netherlands. They have a governance structure in place.
A collaboration agreement between the five partners, LUMC, Radboudumc, Kempenhaeghe-MUMC+, and the two patient organizations Duchenne Parent Project and Spierziekten Nederland, that described the allocation of tasks and responsibilities related to the design, management and use of DDD is drawn and a DDD general board, a DDD advisory board and a structure to access the data is in place.
The DDD general board consists of representatives of each of the five aforementioned partners. The DDD advisory board consist of independent representatives from each of the five DCN partners. The advisory board is a consultative body, making solicited and unsolicited recommendations on scientifically relevant aspects regarding the purpose and use of DDD3, and evaluates data requests from researchers to provide an advice to the DDD general board.
To gain access to data, any researcher, regardless whether they are employed by one of the partners of Duchenne CN, or employed elsewhere, has to provide a written application study rationale, research question(s), study design and methodology.
DDD registry team
Contact: Registration@duchennecentrum.nl